Students in grades 6-8 participated in a 6 week water monitoring project at Liliuokalani, Wailea Peninsula. Students collected field data on weather parameters using an anemometer. We discussed how weather patterns affect different parts of the Hilo Bay watershed.
The Green Standard Water Monitoring Kit enables students to:
- study the relationship between land -use and water quality
- design, implement, and analyze a scientific investigation
- measure and collect data on the physical, chemical, and biological parameters of a river or stream within a local watershed
- identify and explore community issues impacting a local watershed
- develop and evaluate actions toward both strengthening the community stewardship and improving the equality of the local watershed
The Green standard water monitoring kit uses an inquiry based approach to education. This creative process that involves students in all aspects of a scientific investigation. Students ask questions, devise a hypothesis, develop and perform an experiment, analyze the results, draw a conclusion, share their results with others, and take action. With this kit, students are able to explore a wide range of issues regarding the health of their local environment and discover the relationship between land-use and water quality. The outline for conducting a water quality study at Liliuokalani follows:
Step 1: Create a study design
a. identify your watershed: Hilo Bay
b. create a list of goals: basic water quality tests
c. choose freshwater site: Liliuokalani, Wailea Peninsula
Step 2: Conduct study
a. site analysis: map of site and land uses
b. water quality testing: dissolved oxygen, nitrate, pH, phosphate, turbidity
Step 3: Analyze the data
Step 4: Take action
Step 5: Evaluate river study
Goals
students will strengthen observational, analytical and problem solving skills. Students will analyze and compare water quality data. Students will develop an awareness and responsibility to their watershed. Students will become more familiar with the ecosystem. They will also develop a better understanding of the relationship between land use and water quality.
We will conduct a water quality standards study, and compare results with standards.
Test used to analyze water quality include pH, Phosphate, Dissolved oxygen, Nitrate and Turbidity.
For our study, we collected samples from one site at Liliuokalani from November 9th till December 9th.
Each student mapped the field site from an Ariel view, labeling areas of land-use, geological and physical features. We discussed how land-use such as industrial areas, agriculture areas, roadways, communities, and urban areas can all affect water quality. For Liliuokalani, water quality may possibly be affected by the golf course, bathrooms, Banyan Drive, wailea river, etc.
Average of class data estimates a 11-40% bare soil, for a good score.
Average of class data estimates bank erosion to be in a small number of places, for a good score.
Students practiced wafting, in order to evaluate water and soil odor. Samples were collected, students smelled sample, and discussed odor. It was generally agreed that the water had no unusual smell. This is not necessarily an indicator of clean water or soil. Many pesticides and herbicides from agriculture runoff are odorless and colorless, as are many chemical discharged by industry. The soil was pretty stinky, most likely caused by decaying plants and algae.
The appearance of the water can be used as an indicator of water quality and local land-use. The water usually appears a greenish color, which indicates the growth of algae. This is an indicator of high levels of nutrient pollution, which can come from organic wastes, fertilizers or untreated sewage. Other days the water appears more of a more cloudy brown, which indicates elevated levels of suspended sediments. Erosion due to rainfall is most likely the cause of increased sediments in the water.
Water Quality
Dissolved Oxygen
Aquatic animals need Dissolved Oxygen to live. Fish, invertebrates, plants, and anaerobic bacteria all require oxygen for respiration. Oxygen dissolves readily into water from the atmosphere until the water is saturated. Once dissolved in water, the oxygen diffuses very slowly and distribution depends on the movement of aerated water. Oxygen is also produced by aquatic plants, algae, and phytoplankton as a by-product of photosynthesis. The amount of oxygen required varies according to species and stage of life. DO levels below 3 ppm are stressful to most aquatic organisms. DO levels below 2 or 1 ppm will not support fish. Levels of 5 to 6 ppm are usually required for growth and activity.
Dissolved Oxygen Percent Saturation is an important measurement of water quality. Cold water can hold more DO than warm water. For example, water at 28 degrees C will be 100% saturated with 8 ppm DO. However water at 8 degrees C can hold up to 12 ppm of oxygen before its 100% saturated, High levels of bacteria from sewage pollution or large amounts of rotting plants can cause the percent saturation to decrease. This can cause large fluctuations in DO levels throughout the day, which can affect the ability of plants and animals to survive.
The average water temperature at Liliuokalani is 25 degrees C. The water quality test resulted in 8 ppm on average. This means that the DO has a 91-110 % saturation, resulting in an excellent score.
Nitrate
Nitrogen is a nutrient that acts as a fertilizer for aquatic plants. When nutrient levels are high, excessive plant and algae growth creates water quality problems. Nitrogen enters the water from human and animal waste, decomposing organic matter, and run-off of fertilizer from lawns and crops. Nitrogen occurs in water as nitrate, nitrite and ammonium.
Unpolluted waters usually have a nitrate level below 4 ppm. Nitrate levels above 40 ppm are considered unsafe for drinking. The water quality test resulted in 0 ppm nitrate. This is a good score, indicating the water is unpolluted.
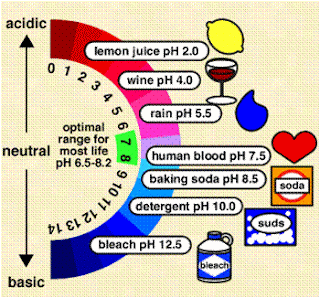
pH
The pH test is one of the most common analysis in water testing. pH is a measurement of the activity of hydrogen ions in a water sample. The pH scale ranges from 0 to 14. Water sample with a a pH below 7.0 are considered acidic, those above 7.0 are basic, with 7.0 considered neutral. A pH range of 6.5 to 8.2 is optimal for most organisms. Rapidly growing algae and vegetation remove carbon dioxide from the water during photosynthesis. This can result in a significant increase in pH. Most natural waters have a pH of 5.5 to 8.5. Acidic, freshly fallen rain water may have a pH of 5.5 to 6.0. Alkaline soils and minerals that can raise pH to 8.0 to 8.5. Sea water usually has a pH value close to 8.0.
The water quality test consistently resulted in a pH reading of 7. This is an excellent score.
Phosphate
Phosphate is a nutrient that acts as a fertilizer for aquatic plants. When nutrient levels are high, excessive plant and algae growth creates water quality problems. Phosphorus occurs in natural waters in the form of phosphate. Over half the phosphate in lakes, streams and rivers comes from detergent.
The water quality tests resulted in a phosphate level of 4 ppm, which is a fair score.
Possible sources of phosphate contributing to high levels may include fertilizers applied to Liliuokalani gardens and the golf course across the street. Further investigation needs to be done to understand the source of phosphate, and effect on the aquatic ecosystem.
Turbidity
Turbidity is the measurement of the relative clarity of water. Turbid water is caused by suspended and colloidal matter such as clay, salt, organic and inorganic matter, and microscopic organisms. Turbidity should not be confused with color, since darkly colored water can still be clear and not turbid. Turbid water may be the result of soil erosion, urban run-off, algal blooms, and bottom sediment disturbances which can be caused by boat traffic and abundant bottom feeders. Students each made their own observations for turbidity. The average class result was a turbidity reading of 100 JTU, for a good score.
Our results from the water quality test came back with good scores, phosphate being the exception with a fair score. It would be interesting to investigate the type of fertilizer that is used on Wailea Peninsula. Our hypothesis is that the fertilizer used by the golf course and Liliuokalani gardens is phosphate based.